Type III Secretion System: Yops
Y. pestis resists host primary immune defense by inhibiting their own uptake by phagocytes. For example, the yopH protein is an essential protein of the Ysc-Yop secretion system. The yopH protein (EC 3.1.3.48) is a protein tyrosine phosphatase (Figure 1).1 Its chaperone is SycH, which recognizes an N-terminal region of the protein and binds to the YopH protein.2 Protein tyrosine phosphatases and tyrosine kinases modulate levels of protein tyrosine phosphorylation.3 Protein tyrosine phosphorylation is an important event in the signal transduction pathway and a key factor in cell cycle regulation. 3 The yopH protein binds a phosphohexapeptide and the key residues involved in hydrogen bonding with the substrate include arginine 230, aspartate 231, arginine 404, cysteine 403, arginine 409, and glycine 408 (Figure 2).4
Through dephosphorylation of tyrosine phosphate proteins in macrophages, yopH prevents phagocytosis. 5,6 Dephosphorylation targets in macrophages include signaling molecules Fak and p130Cas, which inhibits bacterial uptake by macrophages.7,8 In addition to its antiphagocytic activity, the yopH protein plays a role in destabilizing actin filaments.9 The yopH protein dephosphorylates focal adhesion proteins, causing the disruption in focal adhesion complexes and actin filaments.9 The yopH protein is required throughout the infectious process.10
The Ysc-Yop system injects cytokines into host cells; this has been demonstrated in macrophages and epithelial cells.11,12
An example of such a cytokine is yopE. The yopE protein is cytotoxic to HeLa (epithelial) cells.12 Its chaperone is SycE (yerA), which binds to the protein to assist in its secretion and to protect it from intrabacterial degradation.2,13 Unlike yopH, yopE functions mostly at an early step of Y. pestis infection.10 During an early step of Y. pestis infection, yopH and yopE work in concert to prevent phagocytosis by macrophages.12 In addition to its role in preventing phagocytosis, yopE disrupts actin microfilaments through an indirect process, causing a collapse of the host cell cytoskeleton.10 The yopE protein functions as a GTPase-activating protein (GAP).14 The GAP domain occurs at residues 90-219 of the protein.14 The yopEGAP domain resembles the structure of GAP domains from Pseudomonas aeruginosa and Salmonella enterica, based on the structural similarity there are likely few if any conformational changes occur when the yopEGAP domain interacts with the G protein targets.14
The yopE protein acts on the Rho family of GTP-binding proteins, including RhoA, Rac1, and Cdc42 (Figure 3). The Rho family of GTPases are regulators of actin cytoskeleton dynamics.15 Rho family GTPases cycle between active (GTP-bound) and inactive (GDP-bound) as they perform their GTPase activity.14 The yopE protein downregulates Rho family GTPases.9 The downregulation of GTPases causes disruption of actin filaments.14
Another cytokine that works on Rho family GTPases is yopT.16 Its chaperone is SycT protein.16 The yopT protein removes the geranylgeranylated C-terminal cysteine, causing release of Rho family GTPases from the cell membrane.17 When the Rho family GTPases are not located in the membrane, actin fibers cannot form and overall cytoskeleton rearrangements are inhibited.17
Some Ysc-Yop secreted proteins required for later stage infection include ypkA (yopO) and yopM. The yopO protein (EC 2.7.11.1) is a serine/threonine protein kinase.19 It cannot cause apoptosis, on its own, but enhances the apoptotic activity of the yopJ protein.20 The yopM protein shares significant homology with the thrombin-binding domain of the human platelet membrane glycoprotein.21 The yopM protein inhibits thrombin-induced platelet aggregation and is necessary for the virulence of Y. pestis.22 Specifically, the yopM protein binds to caspase-1 to stop inflammasome assembly and activation.23 The yopM protein travels to the nucleus of the target cell.
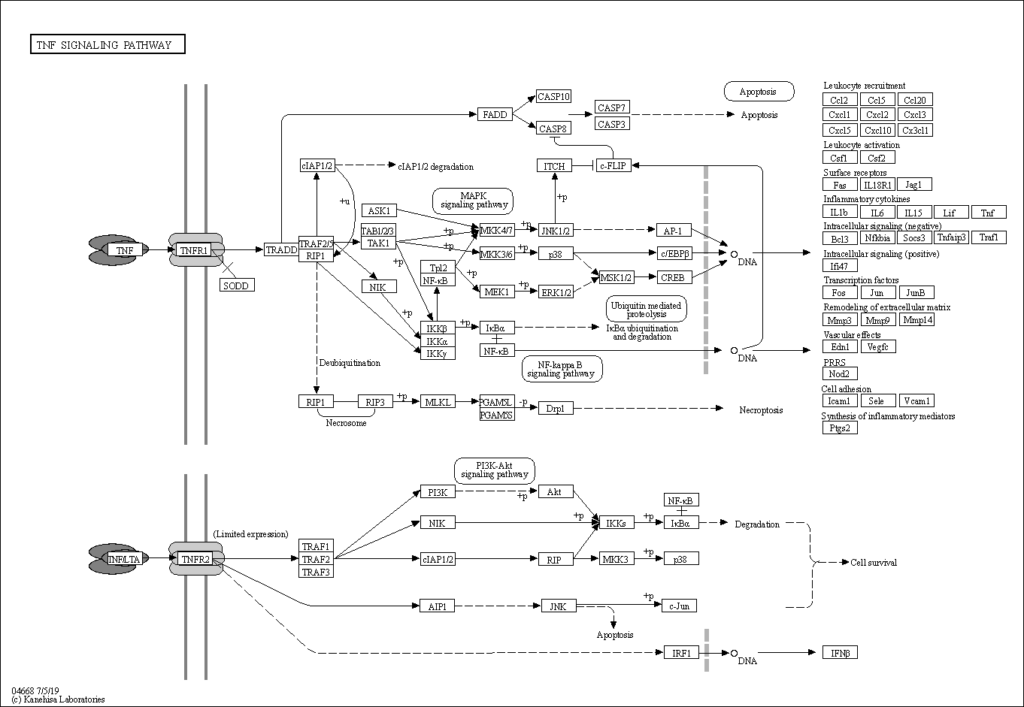
The yopP (yopJ) protein triggers apoptosis of macrophages.20 and prevents the release of tumor necrosis factor-alpha (TNFa). Typically, the lipolysaccharide (LPS) of bacterial cells interact with LPS-binding proteins (LBP), bind to the CD14 receptor on immune cells, stimulate tyrosine kinases and stimulate TNFa production.24 Within the TNF signaling pathway (Figure 4), yopJ downregulate the JNK and p38 proteins. 24 The JNK and map38 proteins are mitogen-activated protein kinase (MAPK). When these proteins are double phosphorylated, they translocate to the nucleus and activate transcription factors, including TNFa which leads to necrosis and apoptosis25 In summary, the yopP protein downregulates the inflammatory response.
Y. pestis has peculiar requirements for calcium. Typically, bacterial and eukaryotic cells have low intracellular concentrations of calcium ions.27 Y. pestis requires a high concentration of calcium when grown at 37C, the temperature of typical mammalian hosts. The yopN protein senses the calcium concentration and thus controls the calcium-regulated expression of yops. When calcium is present in low concentrations, the yopN protein serves as a stop valve in the Ysc injectisome. High concentrations of calcium allow for the removal of the yopN stop valve and allow the injectisome to secret its cytotoxic proteins.
Y. pestis contains a plasmid that codes for pesticin, pesticin immunity, plasminogen activator, and coagulase activities28. On the plasmid, the pla gene encodes for the plasminogen activator, which results in increased fibrin degradation28 The pla surface protease allows the bacteria to disseminate from the primary infection site so that it can infect deep tissue.29. The pla protein allows The plasmid also produces pesticin (pst gene) and a protein that confers immunity for pesticin by lysis of fibrin clots by plasminogen activation.29 Pesticin is a bacteriocin, a peptidic toxin that inhibits the growth of similar or closely related strains.30 Pesticin inhibits DNA synthesis and degrades RNA, but has little effect on protein synthesis,30,31 Likely, pesticin inactivates 6-phosphogluconic acid dehydrogenase to kill the bacteria.32 Although these proteins are not secreted by the Ysc-yop secretion system, they are essential for the dissemination of the bacteria and promote its survival over other possible pathogens.
References
(1) EC 3.1.3.48 – protein-tyrosine-phosphatase – BRENDA Enzyme Database https://brenda-enzymes.info/enzyme.php?ecno=3.1.3.48 (accessed 2022 -04 -28).
(2) Wattiau, P.; Woestyn, S.; Cornelis, G. R. Customized Secretion Chaperones in Pathogenic Bacteria. Mol. Microbiol. 1996, 20 (2), 255–262. https://doi.org/10.1111/j.1365-2958.1996.tb02614.x.
(3) Guan, K.; Dixon, J. E. Protein Tyrosine Phosphatase Activity of an Essential Virulence Determinant in Yersinia. Science 1990, 249 (4968), 553–556. https://doi.org/10.1126/science.2166336.
(4) Phan, J.; Lee, K.; Cherry, S.; Tropea, J. E.; Burke, Terrence R.; Waugh, D. S. High-Resolution Structure of the Yersinia Pestis Protein Tyrosine Phosphatase YopH in Complex with a Phosphotyrosyl Mimetic-Containing Hexapeptide. Biochemistry 2003, 42 (45), 13113–13121. https://doi.org/10.1021/bi030156m.
(5) Bliska, J. B.; Clemens, J. C.; Dixon, J. E.; Falkow, S. The Yersinia Tyrosine Phosphatase: Specificity of a Bacterial Virulence Determinant for Phosphoproteins in the J774A.1 Macrophage. J. Exp. Med. 1992, 176 (6), 1625–1630. https://doi.org/10.1084/jem.176.6.1625.
(6) Andersson, K.; Carballeira, N.; Magnusson, K.-E.; Persson, C.; Stendahl, O.; Wolf-Watz, H.; Fällman, M. YopH of Yersinia Pseudotuberculosis Interrupts Early Phosphotyrosine Signalling Associated with Phagocytosis. Mol. Microbiol. 1996, 20 (5), 1057–1069. https://doi.org/10.1111/j.1365-2958.1996.tb02546.x.
(7) Identification of P130Cas as a Substrate of Yersinia YopH (Yop51), a Bacterial Protein Tyrosine Phosphatase That Translocates into Mammalian Cells and Targets Focal Adhesions. EMBO J. 1997, 16 (10), 2730–2744. https://doi.org/10.1093/emboj/16.10.2730.
(8) The PTPase YopH Inhibits Uptake of Yersinia, Tyrosine Phosphorylation of P130Cas and FAK, and the Associated Accumulation of These Proteins in Peripheral Focal Adhesions. EMBO J. 1997, 16 (9), 2307–2318. https://doi.org/10.1093/emboj/16.9.2307.
(9) Black, D. S.; Bliska, J. B. The RhoGAP Activity of the Yersinia Pseudotuberculosis Cytotoxin YopE Is Required for Antiphagocytic Function and Virulence. Mol. Microbiol. 2000, 37 (3), 515–527. https://doi.org/10.1046/j.1365-2958.2000.02021.x.
(10) Hueck, C. J. Type III Protein Secretion Systems in Bacterial Pathogens of Animals and Plants. Microbiol. Mol. Biol. Rev. 1998, 62 (2), 379–433. https://doi.org/10.1128/MMBR.62.2.379-433.1998.
(11) Portnoy, D. A.; Moseley, S. L.; Falkow, S. Characterization of Plasmids and Plasmid-Associated Determinants of Yersinia Enterocolitica Pathogenesis. Infect. Immun. 1981, 31 (2), 775–782. https://doi.org/10.1128/iai.31.2.775-782.1981.
(12) Rosqvist, R.; Forsberg, Å.; Rimpiläinen, M.; Bergman, T.; Wolf-Watz, H. The Cytotoxic Protein YopE of Yersinia Obstructs the Primary Host Defence. Mol. Microbiol. 1990, 4 (4), 657–667. https://doi.org/10.1111/j.1365-2958.1990.tb00635.x.
(13) Forsberg, A.; Wolf-Watz, H. Genetic Analysis of the YopE Region of Yersinia Spp.: Identification of a Novel Conserved Locus, YerA, Regulating YopE Expression. J. Bacteriol. 1990, 172 (3), 1547–1555. https://doi.org/10.1128/jb.172.3.1547-1555.1990.
(14) Evdokimov, A. G.; Tropea, J. E.; Routzahn, K. M.; Waugh, D. S. Crystal Structure of the Yersinia Pestis GTPase Activator YopE. Protein Sci. Publ. Protein Soc. 2002, 11 (2), 401–408. https://doi.org/10.1110/ps.34102.
(15) Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279 (5350), 509–514. https://doi.org/10.1126/science.279.5350.509.
(16) Iriarte, M.; Cornelis, G. R. YopT, a New Yersinia Yop Effector Protein, Affects the Cytoskeleton of Host Cells. Mol. Microbiol. 1998, 29 (3), 915–929. https://doi.org/10.1046/j.1365-2958.1998.00992.x.
(17) Aepfelbacher, M.; Zumbihl, R.; Heesemann, J. Modulation of Rho GTPases and the Actin Cytoskeleton by YopT of Yersinia. In Bacterial Virulence Factors and Rho GTPases; Boquet, P., Lemichez, E., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin, Heidelberg, 2005; pp 167–175. https://doi.org/10.1007/3-540-27511-8_9.
(18) KEGG PATHWAY: Yersinia infection – Homo sapiens (human) https://www.kegg.jp/kegg-bin/show_pathway?hsa05135 (accessed 2022 -04 -29).
(19) KEGG ENZYME: 2.7.11.1 https://www.genome.jp/entry/2.7.11.1 (accessed 2022 -04 -28).
(20) Monack, D. M.; Mecsas, J.; Ghori, N.; Falkow, S. Yersinia Signals Macrophages to Undergo Apoptosis and YopJ Is Necessary for This Cell Death. Proc. Natl. Acad. Sci. 1997, 94 (19), 10385–10390. https://doi.org/10.1073/pnas.94.19.10385.
(21) Leung, K. Y.; Straley, S. C. The YopM Gene of Yersinia Pestis Encodes a Released Protein Having Homology with the Human Platelet Surface Protein GPIb Alpha. J. Bacteriol. 1989, 171 (9), 4623–4632. https://doi.org/10.1128/jb.171.9.4623-4632.1989.
(22) Leung, K. Y.; Reisner, B. S.; Straley, S. C. YopM Inhibits Platelet Aggregation and Is Necessary for Virulence of Yersinia Pestis in Mice. Infect. Immun. 1990, 58 (10), 3262–3271.
(23) Demeure, C. E.; Dussurget, O.; Mas Fiol, G.; Le Guern, A.-S.; Savin, C.; Pizarro-Cerdá, J. Yersinia Pestis and Plague: An Updated View on Evolution, Virulence Determinants, Immune Subversion, Vaccination, and Diagnostics. Genes Immun. 2019, 20 (5), 357–370. https://doi.org/10.1038/s41435-019-0065-0.
(24) Palmer, L. E.; Hobbie, S.; Galán, J. E.; Bliska, J. B. YopJ of Yersinia Pseudotuberculosis Is Required for the Inhibition of Macrophage TNF-α Production and Downregulation of the MAP Kinases P38 and JNK. Mol. Microbiol. 1998, 27 (5), 953–965. https://doi.org/10.1046/j.1365-2958.1998.00740.x.
(25) Ulevitch, R. J.; Tobias, P. S. Receptor-Dependent Mechanisms of Cell Stimulation by Bacterial Endotoxin. Annu. Rev. Immunol. 1995, 13 (1), 437–457. https://doi.org/10.1146/annurev.iy.13.040195.002253.
(26) KEGG PATHWAY: TNF signaling pathway – Homo sapiens (human) https://www.kegg.jp/pathway/hsa04668+7124 (accessed 2022 -04 -29).
(27) Forsberg, Å.; Viitanen, A.-M.; Skurnik, M.; Wolf-Watz, H. The Surface-Located YopN Protein Is Involved in Calcium Signal Transduction in Yersinia Pseudotuberculosis. Mol. Microbiol. 1991, 5 (4), 977–986. https://doi.org/10.1111/j.1365-2958.1991.tb00773.x.
(28) Sodeinde, O. A.; Sample, A. K.; Brubaker, R. R.; Goguen, J. D. Plasminogen Activator/Coagulase Gene of Yersinia Pestis Is Responsible for Degradation of Plasmid-Encoded Outer Membrane Proteins. Infect. Immun. 1988, 56 (10), 2749–2752.
(29) Sodeinde, O. A.; Subrahmanyam, Y. V. B. K.; Stark, K.; Quan, T.; Bao, Y.; Goguen, J. D. A Surface Protease and the Invasive Character of Plague. Science 1992, 258 (5084), 1004–1007.
(30) Elgat, M.; Ben-Gurion, R. Mode of Action of Pesticin. J. Bacteriol. 1969, 98 (2), 359–367.
(31) Brubaker, R. R.; Surgalla, M. J. PESTICINS II. I and II. J. Bacteriol. 1962, 84 (3), 539–545.(32) Eisler, D. M.; Heckly, R. J. Possible Mechanisms of Action of an Anti-Pasteurella Pestis Factor1. J. Bacteriol. 1968, 96 (6), 1977–1981.
Hi Lindsey, very interesting read! It’s fascinating how many proteins are involved in Y. pestis’ virulence. If my understanding is correct, the overall function of Y. pestis’ injectisome is to combat the host’s immune response, based on two of the primary actions of the yop proteins (shutting down the attacking phagocytes and downregulating the inflammatory response).
In addition to the innate phagocytic response described in your Healthy and Disease States page, phagocytes are also involved in adaptive immunity, such as pathogen opsonization by antibodies. Do the yops have the same destructive effect on phagocytes regardless of whether it’s an innate or an adaptive response? What’s the difference, if any?
The yops have the destructive effects mainly in the innate phagocytic response.
The host must develop a strong adaptive immune response to clear the infection. But even this response can be overcome by the yops. The yops, specifically YopP inhibits the priming of the specific CD8 T cell response. (1)
(1) Heesemann, J.; Sing, A.; Trülzsch, K. Yersinia’s Stratagem: Targeting Innate and Adaptive Immune Defense. Current Opinion in Microbiology 2006, 9 (1), 55–61. https://doi.org/10.1016/j.mib.2005.10.018.
Thank you for such an informative site! You mention that “during an early step of Y. pestis infection, yopH and yopE work in concert to prevent phagocytosis by macrophages.” Could you explain a bit more about how yopH & E work together — are they influencing each other, or is it that they’re doing different work simultaneously? In effect, I’m wondering what this “concert” between yopH & E looks like?
YopH disrupts the homeostasis of tyrosine phosphorylation by dephosphorylating proteins: p130Cas, FAK, paxillin, Lck, Fyb and SKAP-HOM. (1)
YopE causes activation of small G-proteins including Rac, RhoA,and CDC42. (1)
All of these proteins are involved in the regulation of the actin cytoskeleton. So, the prevent phagocytosis by regulating the actin polymerization in macrophages, thereby preventing the macrophages from engulfing the bacteria.
(see this KEGG pathway: https://www.genome.jp/pathway/map04510 )
(1) Trosky, J. E.; Liverman, A. D. B.; Orth, K. Yersinia Outer Proteins: Yops. Cellular Microbiology 2008, 10 (3), 557–565. https://doi.org/10.1111/j.1462-5822.2007.01109.x.